BIOVIC is dedicated to crafting exceptional, quality products meticulously tailored to meet the unique needs of patients in the communities we serve, empowering better health and brighter futures.
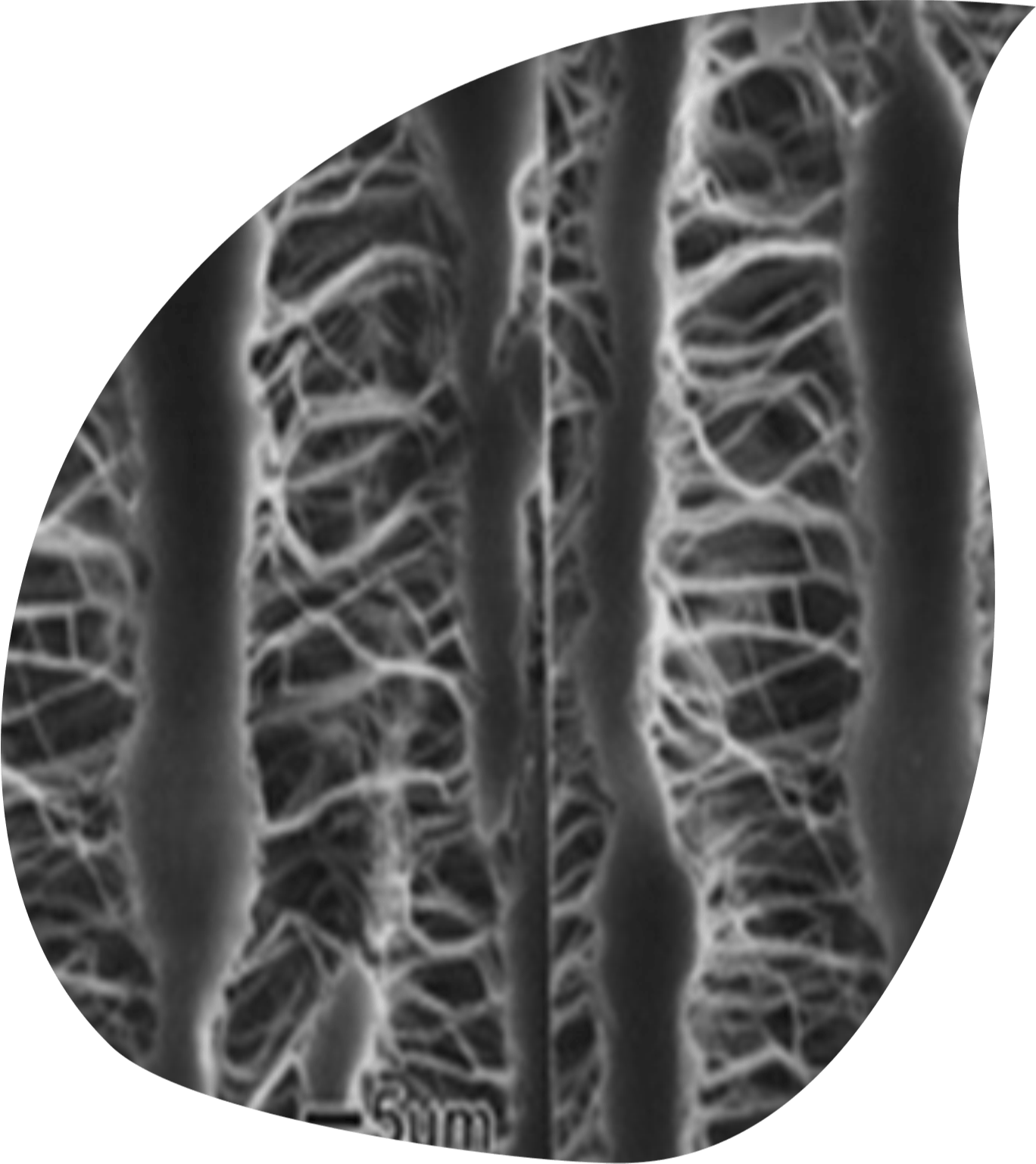
Polytetrafluoroethylene (PTFE) is a fully fluorinated polymer renowned for its exceptional chemical resistance, high-temperature stability, and remarkable non-stick properties. ePTFE is "expanded" PTFE or the porous form of PTFE, although the terms ePTFE and PTFE are often used interchangeably. As an inert, lubricious material, ePTFE has stood the test of time, maintaining its status as the gold standard for synthetic blood vessel substitutes after more than 40 years of clinical use. ePTFE vascular grafts offer a vital option for bypassing or replacing diseased or damaged veins and arteries. Their highly porous structure promotes tissue ingrowth, ensuring superior integration with the body. To this day, ePTFE grafts remain the dominant choice in arteriovenous access and small-caliber synthetic graft applications, continuing to set the benchmark for innovation and reliability in vascular care.
Introducing BIOVIC’s flagship innovation, the AVATAR® ePTFE Vascular Graft—a groundbreaking solution for arterial vascular reconstruction, segmental bypass, and arteriovenous vascular access. Crafted from ePTFE, a biomaterial with a proven and reliable history in clinical applications, the AVATAR® graft sets a new standard in vascular care.
Engineered by BIOVIC’s expert team using our proprietary Humidity Resistant Technology (HRT), this graft not only meets but exceeds the performance and handling characteristics of its competitors. Its unique open, porous microstructure on both internal and external surfaces promotes optimal tissue ingrowth and accelerates the body’s natural healing response.
Manufactured under the most stringent quality control conditions, the AVATAR® graft is several times stronger than vein tissue while remaining soft, flexible, and fully conformable. With a full range of sizes to suit diverse patient needs, the AVATAR® ePTFE Vascular Graft is designed to deliver exceptional results and redefine the future of vascular surgery.
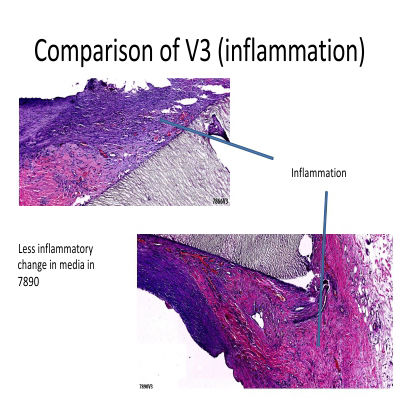
In pre-clinical studies, the AVATAR® Vascular Graft underwent rigorous evaluation using a dialysis graft shunt model, where it was compared alongside leading competitor grafts. Histological analyses revealed that the AVATAR® graft not only performed on par with its competitors but, in several key areas, demonstrated superior results, setting a new benchmark for excellence in vascular care.
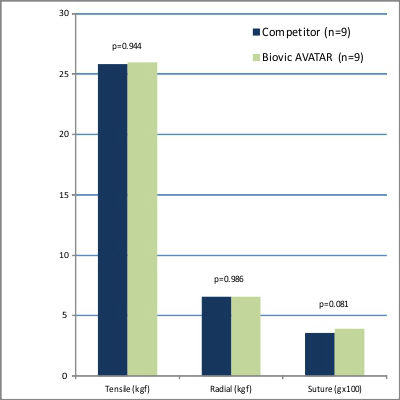
In comprehensive comparative testing, the AVATAR® Vascular Graft was evaluated against leading commercially available competitors in terms of strength and handling. The results confirmed that the AVATAR® graft was equivalent to its counterparts across all critical parameters. Additionally, feedback from clinical evaluations highlighted that the AVATAR® graft offered superior softness and enhanced handling, setting it apart as a preferred choice for surgeons seeking exceptional performance and precision.
The AVATAR® ePTFE Vascular Graft is meticulously packaged in dual barrier sterile packaging to ensure the highest standards of safety and reliability. With more than 50 product codes available, it offers a comprehensive range of options to meet the diverse needs of patients and clinicians alike.
Please click the link button to view the full document.